
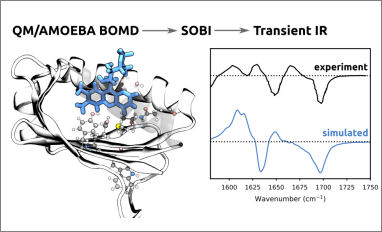
 Congratulations to our PhD student Michele Nottoli for his publication entitled: Energy, Structures, and Response Properties with a Fully Coupled QM/AMOEBA/ddCOSMO Implementation. In this work, we present the implementation of a fully coupled polarizable QM/MM/continuum model based on the AMOEBA polarizable force field and the domain decomposition implementation of the conductor-like screening model.
Congratulations to our PhD student Michele Nottoli for his publication entitled: Energy, Structures, and Response Properties with a Fully Coupled QM/AMOEBA/ddCOSMO Implementation. In this work, we present the implementation of a fully coupled polarizable QM/MM/continuum model based on the AMOEBA polarizable force field and the domain decomposition implementation of the conductor-like screening model.
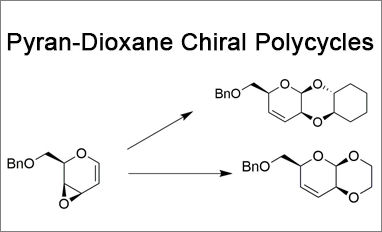 We present two novel linear fused pyran-dioxane based bi- and tricycles, synthesized with total stereoselectivity from a glycal derived vinyl epoxide. Chiral heteropolycyclic structures are widespread in compounds of high pharmaceutical relevance. In particular, linear fused pyran-dioxane based polycycles can be found in several naturally occurring molecules, and among them, cardiac glycosides and antibiotic spectinomycin are characterized by a cis–cisoid–trans geometry.
We present two novel linear fused pyran-dioxane based bi- and tricycles, synthesized with total stereoselectivity from a glycal derived vinyl epoxide. Chiral heteropolycyclic structures are widespread in compounds of high pharmaceutical relevance. In particular, linear fused pyran-dioxane based polycycles can be found in several naturally occurring molecules, and among them, cardiac glycosides and antibiotic spectinomycin are characterized by a cis–cisoid–trans geometry.
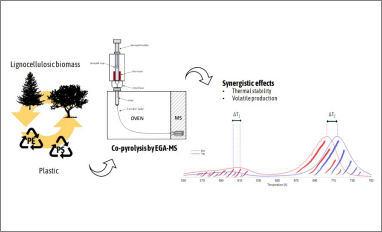 Congratulations to our Ph.D. student Federica Nardella for her publication entitled "Co-pyrolysis of wood and plastic: Evaluation of synergistic effects and kinetic data by evolved gas analysis-mass spectrometry (EGA-MS)". Co-pyrolysis of biomass and plastics gives rise to synergistic effects that can improve the properties of the resulting bio-oil. In this paper, the co-pyrolysis of lignocellulose (softwoods and hardwoods) and plastic (polyethylene and polystyrene) mixtures at different ratios was investigated by evolved gas analysis-mass spectrometry (EGA-MS).
Congratulations to our Ph.D. student Federica Nardella for her publication entitled "Co-pyrolysis of wood and plastic: Evaluation of synergistic effects and kinetic data by evolved gas analysis-mass spectrometry (EGA-MS)". Co-pyrolysis of biomass and plastics gives rise to synergistic effects that can improve the properties of the resulting bio-oil. In this paper, the co-pyrolysis of lignocellulose (softwoods and hardwoods) and plastic (polyethylene and polystyrene) mixtures at different ratios was investigated by evolved gas analysis-mass spectrometry (EGA-MS).
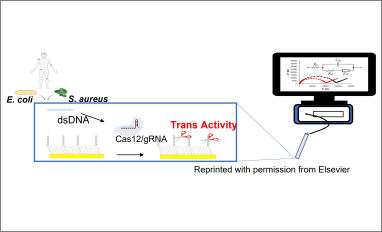 Congratulations to our Ph.D. students Andrea Bonini, Noemi Poma and Federico Vivaldi from the Chemistry Lab for Analytical Technologies and Sensors (CATS), for their recent research article entitled "A label-free impedance biosensing assay based on CRISPR/Cas12a collateral activity for bacterial DNA detection". The rapid and selective identification in the clinical setting of pathogenic bacteria causing healthcare associated infections (HAIs) and in particular blood stream infections (BSIs) is a major challenge.
Congratulations to our Ph.D. students Andrea Bonini, Noemi Poma and Federico Vivaldi from the Chemistry Lab for Analytical Technologies and Sensors (CATS), for their recent research article entitled "A label-free impedance biosensing assay based on CRISPR/Cas12a collateral activity for bacterial DNA detection". The rapid and selective identification in the clinical setting of pathogenic bacteria causing healthcare associated infections (HAIs) and in particular blood stream infections (BSIs) is a major challenge.



