
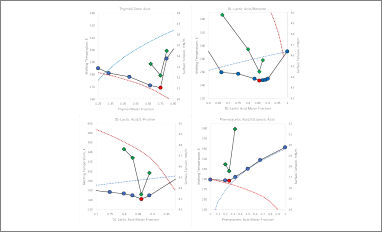
 A new publication, with the contribution of our PhD student Elisa Rossi, dealing with DESs singularities at their eutectic point, especially in terms of ionic conductivity and surface tension. The results of this study underline the "entropic driving force" on DESs formation and show the surface tension as an useful technique for determining the eutectic point.
A new publication, with the contribution of our PhD student Elisa Rossi, dealing with DESs singularities at their eutectic point, especially in terms of ionic conductivity and surface tension. The results of this study underline the "entropic driving force" on DESs formation and show the surface tension as an useful technique for determining the eutectic point.
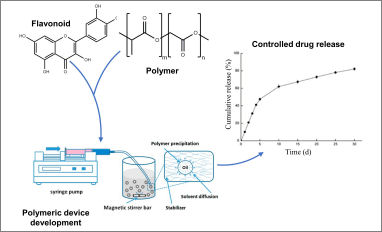 Congratulations to our PhD student Gianni Pecorini for his publication entitled "Polymeric Systems for the Controlled Release of Flavonoids" in Pharmaceutics. In this article the different classes of flavonoids are described together with the polymers most commonly employed for drug delivery applications. Representative drug delivery systems are discussed, highlighting the most common techniques for their preparation. The flavonoids investigated for polymer system encapsulation are then presented with their main source of extraction and biological properties.
Congratulations to our PhD student Gianni Pecorini for his publication entitled "Polymeric Systems for the Controlled Release of Flavonoids" in Pharmaceutics. In this article the different classes of flavonoids are described together with the polymers most commonly employed for drug delivery applications. Representative drug delivery systems are discussed, highlighting the most common techniques for their preparation. The flavonoids investigated for polymer system encapsulation are then presented with their main source of extraction and biological properties.
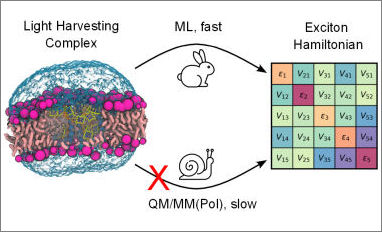 Congratulations to our PhD student Edoardo Cignoni for his publication entitled "Machine Learning Exciton Hamiltonians in Light-Harvesting Complexes" in the Journal of Chemical Theory and Computation. They propose a machine learning (ML)-based strategy for an inexpensive calculation of excitonic properties of light-harvesting complexes (LHCs). The strategy uses classical molecular dynamics simulations of LHCs in their natural environment in combination with ML prediction of the excitonic Hamiltonian of the embedded aggregate of pigments.
Congratulations to our PhD student Edoardo Cignoni for his publication entitled "Machine Learning Exciton Hamiltonians in Light-Harvesting Complexes" in the Journal of Chemical Theory and Computation. They propose a machine learning (ML)-based strategy for an inexpensive calculation of excitonic properties of light-harvesting complexes (LHCs). The strategy uses classical molecular dynamics simulations of LHCs in their natural environment in combination with ML prediction of the excitonic Hamiltonian of the embedded aggregate of pigments.
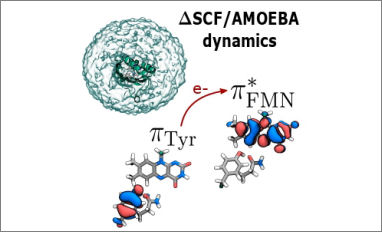 Congratulations to our PhD student Patrizia Mazzeo for her publication entitled "Fast Method for Excited-State Dynamics in Complex Systems and Its Application to the Photoactivation of a Blue Light Using Flavin Photoreceptor". The excited-state dynamics of molecules embedded in complex (bio)matrices is still a challenging goal for quantum chemical models. Hybrid QM/MM models have proven to be an effective strategy, but an optimal combination of accuracy and computational cost still has to be found.
Congratulations to our PhD student Patrizia Mazzeo for her publication entitled "Fast Method for Excited-State Dynamics in Complex Systems and Its Application to the Photoactivation of a Blue Light Using Flavin Photoreceptor". The excited-state dynamics of molecules embedded in complex (bio)matrices is still a challenging goal for quantum chemical models. Hybrid QM/MM models have proven to be an effective strategy, but an optimal combination of accuracy and computational cost still has to be found.



