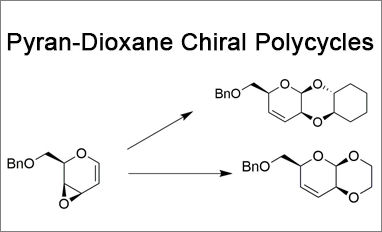
 We present two novel linear fused pyran-dioxane based bi- and tricycles, synthesized with total stereoselectivity from a glycal derived vinyl epoxide. Chiral heteropolycyclic structures are widespread in compounds of high pharmaceutical relevance. In particular, linear fused pyran-dioxane based polycycles can be found in several naturally occurring molecules, and among them, cardiac glycosides and antibiotic spectinomycin are characterized by a cis–cisoid–trans geometry.
We present two novel linear fused pyran-dioxane based bi- and tricycles, synthesized with total stereoselectivity from a glycal derived vinyl epoxide. Chiral heteropolycyclic structures are widespread in compounds of high pharmaceutical relevance. In particular, linear fused pyran-dioxane based polycycles can be found in several naturally occurring molecules, and among them, cardiac glycosides and antibiotic spectinomycin are characterized by a cis–cisoid–trans geometry.
The straightforward methodology described involves a substrate-dependent stereospecific glycosylation step followed by an intramolecular SN2′ conjugate addition process, leading to a pyran-dioxane-cyclohexane tricycle with a cis–cisoid–trans stereochemistry, in agreement with the geometry of many natural products. The stereochemical analysis of these compounds, which was realized by a combined NMR/computational approach, is also reported.
The work is available at the following link: https://doi.org/10.1039/D1OB01541A


