 Stabilized Arylzinc Iodides in Negishi Acylative Cross-Coupling: A Modular Synthesis of Chalcones.
Stabilized Arylzinc Iodides in Negishi Acylative Cross-Coupling: A Modular Synthesis of Chalcones.
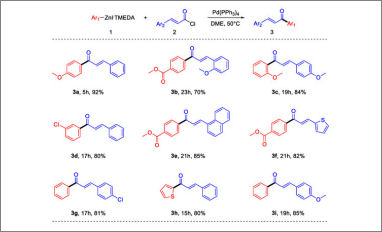
Stabilized arylzinc iodides, synthesized by direct insertion of zinc into the corresponding halides, were used as nucleophiles into an acylative Negishi coupling reaction to synthesize chalcones. The reaction conditions were optimized to afford optimal results on a model reaction and then applied to synthesize nine compounds. Esters, chlorides, electron-rich, electron-poor and sterically hindered substrates are well tolerated and even heteroaryl derivatives can be synthesized.
Full-Text available at https://doi.org/10.3390/org3020006


