 Congratulations to our PhD candidate Andrea Giovanelli for his work entitled “Retro-Favorskii reaction employing fluoride sources and its use as immobilization strategy” published on the Journal of Fluorine Chemistry. Retro-Favorskii is the reaction that converts propargyl alcohols into the alkyne and ketone precursors. This reaction is commonly used to deprotect terminal alkynes with elimination of acetone.
Congratulations to our PhD candidate Andrea Giovanelli for his work entitled “Retro-Favorskii reaction employing fluoride sources and its use as immobilization strategy” published on the Journal of Fluorine Chemistry. Retro-Favorskii is the reaction that converts propargyl alcohols into the alkyne and ketone precursors. This reaction is commonly used to deprotect terminal alkynes with elimination of acetone.
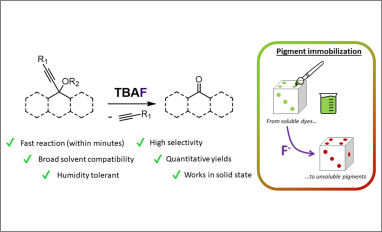
In this study, we developed a novel and simple approach to perform retro-Favorskii type reactions comprising the use of a readily available fluoride source. Compared to the methods usually employed – which rely on the use of strong basic systems, such as KOH in toluene, at high temperatures and a reaction time of several hours – the one we propose employs milder conditions, shorter reaction times, is compatible with more solvents, and produces no appreciable amount of byproducts, thus allowing simple workup procedures. The possibility of performing such reactions in a fast and reliable manner, without the appreciable formation of byproducts, can offer new interesting opportunities to efficiently employ propargyl alcohols derivatives as a masking group for carbonyls. We have applied this approach for the deposition of polymeric latent pigments on HDPE fragments obtained from the glorious Siluròn.
Andrea’s work is available at the following link:https://doi.org/10.1016/j.jfluchem.2025.110405


