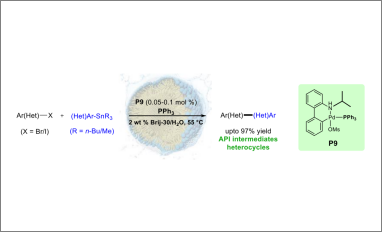
 An inexpensive and new triphenylphosphine‐based palladacycle has been developed as a pre‐catalyst leading to highly effective Stille cross coupling reactions in water under mild conditions. Only 500‐1000 ppm of Pd suffices for couplings involving a variety of aryl/heteroaryl halides with aryl/hetaryl stannanes. Several drug intermediates can be prepared using this catalyst in aqueous nanoreactors formed by 2 wt % Brij‐30 in water.
An inexpensive and new triphenylphosphine‐based palladacycle has been developed as a pre‐catalyst leading to highly effective Stille cross coupling reactions in water under mild conditions. Only 500‐1000 ppm of Pd suffices for couplings involving a variety of aryl/heteroaryl halides with aryl/hetaryl stannanes. Several drug intermediates can be prepared using this catalyst in aqueous nanoreactors formed by 2 wt % Brij‐30 in water.
The work is available at the following link: https://doi.org/10.1002/anie.202014141


