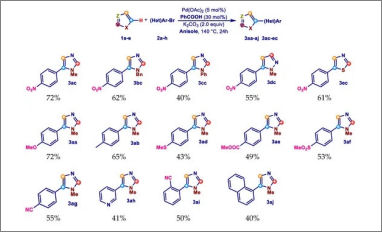
 Congratulations to our Ph.D. student Elisabetta Rosadoni for her publication "Ligandless Palladium-Catalyzed Direct C-5 Arylation of Azoles Promoted by Benzoic Acid in Anisole" on Molecules. In this paper we report a selective C-5 arylation procedure involving anisole as an EHS green reaction solvent. In addition, the beneficial role of benzoic acid in this reaction as an additive is also highlighted. The palladium-catalyzed direct arylation of azoles with (hetero)aryl halides is nowadays one of the most versatile and efficient procedures for the selective synthesis of heterobiaryls.
Congratulations to our Ph.D. student Elisabetta Rosadoni for her publication "Ligandless Palladium-Catalyzed Direct C-5 Arylation of Azoles Promoted by Benzoic Acid in Anisole" on Molecules. In this paper we report a selective C-5 arylation procedure involving anisole as an EHS green reaction solvent. In addition, the beneficial role of benzoic acid in this reaction as an additive is also highlighted. The palladium-catalyzed direct arylation of azoles with (hetero)aryl halides is nowadays one of the most versatile and efficient procedures for the selective synthesis of heterobiaryls.
Although this procedure is of great interest in the industrial field, the wide use of a reaction medium such as DMF or DMA, two polar aprotic solvents coded as dangerous according to environmental, health, safety (EHS) parameters, strongly limits its actual use. In contrast, the use of aromatic solvents as the reaction medium for direct arylations, although some of them show good EHS values, is poorly reported. The article is available at the following link: https://www.mdpi.com/1983426


